Equilibrium Constant K And Q . conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. q and k are used to describe the state of equilibrium in a chemical reaction. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k:
from www.alamy.com
when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. q and k are used to describe the state of equilibrium in a chemical reaction. conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and.
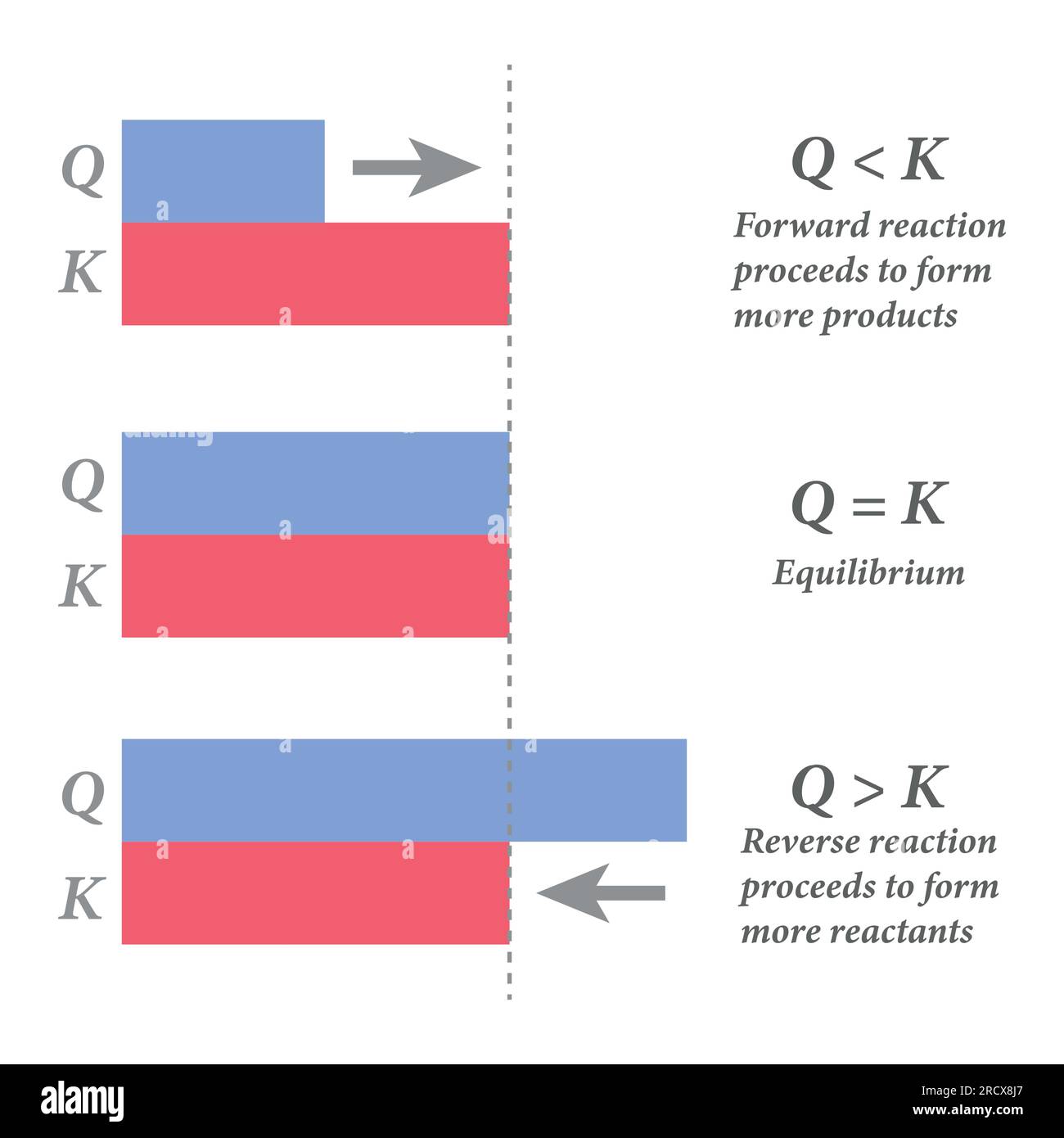
Direction of shift of reaction depending upon the value of Q as
Equilibrium Constant K And Q the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. q and k are used to describe the state of equilibrium in a chemical reaction. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the.
From www.slideserve.com
PPT Thermodynamics Spontaneity, Entropy, and Free Energy PowerPoint Equilibrium Constant K And Q when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. q and k are used to describe the state of equilibrium in a chemical reaction. to determine which direction a reaction. Equilibrium Constant K And Q.
From www.slideserve.com
PPT Review Expressions of the thermodynamic equilibrium constant K Equilibrium Constant K And Q an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: q and k are used to describe the state of equilibrium in a chemical reaction. to determine which direction a reaction will. Equilibrium Constant K And Q.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Equilibrium Constant K And Q the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. to determine which direction. Equilibrium Constant K And Q.
From www.toppr.com
Given the equilibrium constant, Kc of the reaction Cu(s) + 2Ag^ + (aq Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. q and k are used to describe the state of equilibrium in a chemical reaction. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. the constant value of q exhibited by a. Equilibrium Constant K And Q.
From www.slideserve.com
PPT Chemical Equilibrium Q, K, and Calculations Chapter 16 PowerPoint Equilibrium Constant K And Q q and k are used to describe the state of equilibrium in a chemical reaction. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. the constant value of. Equilibrium Constant K And Q.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant K And Q to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. q and k are used to describe the state of equilibrium in a chemical reaction. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: an equilibrium constant calculated from. Equilibrium Constant K And Q.
From www.chegg.com
Solved 22. Write the appropriate equilibrium constant Equilibrium Constant K And Q to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. q and k are used to describe the state of equilibrium in a chemical reaction. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: an equilibrium constant calculated from. Equilibrium Constant K And Q.
From www.youtube.com
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Equilibrium Constant K And Q to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. q and k are used. Equilibrium Constant K And Q.
From www.youtube.com
Equilibrium Constant (K) & Reaction Quotient (Q) YouTube Equilibrium Constant K And Q the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: q and k are used to describe the state of equilibrium in a chemical reaction. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. to determine which direction a reaction. Equilibrium Constant K And Q.
From thechemistrynotes.com
Reaction Quotient (Q) Equation, Calculation, Types, Units Equilibrium Constant K And Q the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. an equilibrium constant calculated from. Equilibrium Constant K And Q.
From www.chegg.com
Solved The equilibrium constant, Kc, for the following Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. q and k are used to describe the state of equilibrium in a chemical reaction. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. to determine which direction a reaction will go towards,. Equilibrium Constant K And Q.
From www.vrogue.co
Chemical Equilibrium I Types Of Equilibrium Equilibri vrogue.co Equilibrium Constant K And Q an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. q and k are used to describe the state of equilibrium in a chemical reaction. the constant value of q exhibited. Equilibrium Constant K And Q.
From www.youtube.com
Reaction Quotient (K) and Equilibrium Constant (K) Problems & Examples Equilibrium Constant K And Q an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. to determine which direction a reaction will. Equilibrium Constant K And Q.
From www.youtube.com
Relating Equilibrium Constants Kp and Kc (Keq) YouTube Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. q and k are used to describe the state of equilibrium in a chemical reaction. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. when equilibrium is achieved, the concentrations of reactants and. Equilibrium Constant K And Q.
From www.chegg.com
Solved The equilibrium constant, Kc, for the following Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. q and k are. Equilibrium Constant K And Q.
From askfilo.com
Relationship between Equilibrium Constant K Reaction Quotient (Q) and Gib.. Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. q and k are used to describe the state of equilibrium in a chemical reaction. the constant value of q exhibited by a system at equilibrium is called the equilibrium constant, k: an equilibrium constant calculated from partial pressures (\(k_p\)) is. Equilibrium Constant K And Q.
From artofsmart.com.au
Guide to HSC Chemistry Module 5 Equilibrium and Acid Reactions Equilibrium Constant K And Q conversion between a value for \(k_c\), an equilibrium constant expressed in terms of concentrations, and. when equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of qc. q and k are used to describe the state of equilibrium in a chemical reaction. an equilibrium constant calculated from partial pressures (\(k_p\)). Equilibrium Constant K And Q.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Equilibrium Constant K And Q an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. q and k are used to describe the state of equilibrium in a chemical reaction. to determine which direction a reaction will go towards, simply compare \(q_c\), the initial concentration ratio, to \(k_c\), the. conversion between a value for. Equilibrium Constant K And Q.